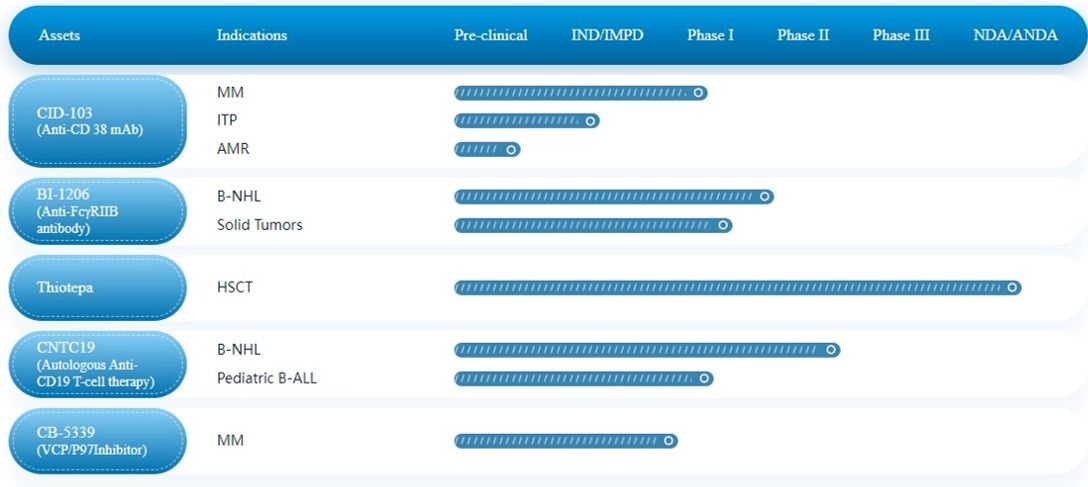
TECHNOLOGY
CASI Has a Promising and Fast-Advancing Pipeline with AMR and ITP as Lead Disease Indications
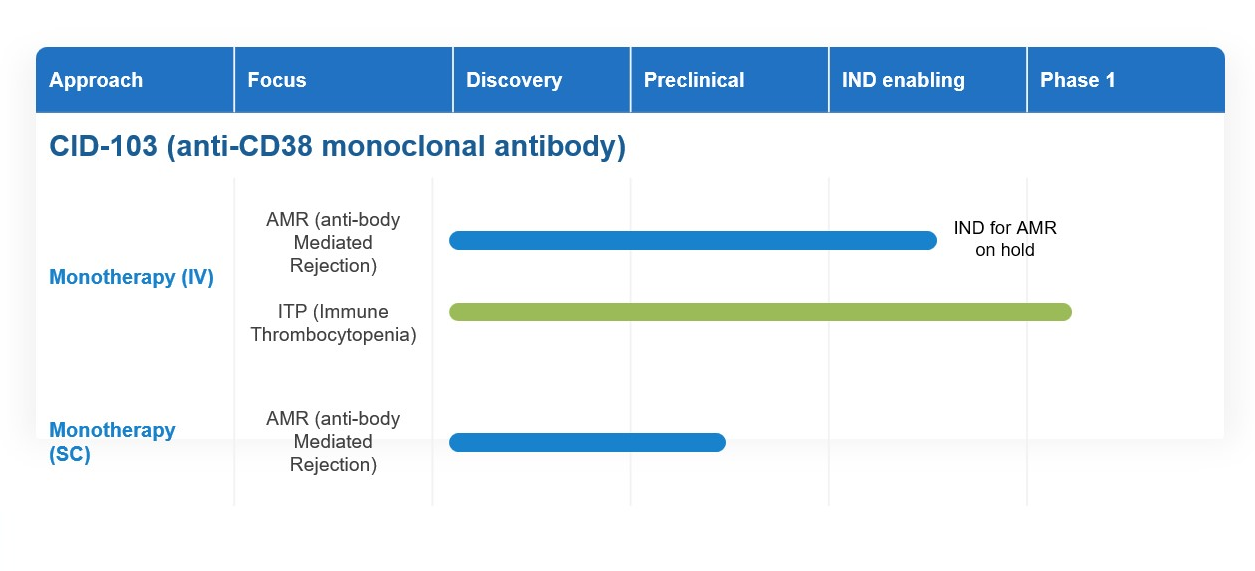
At CASI, we are harnessing cutting-edge research and technology to develop a new therapeutic option for antibody-mediated rejection (AMR) and autoimmune diseases. Today, our work is focused on a lead drug candidate, CID-103. CID-103 is an investigational anti-CD38 monoclonal antibody.
The Anti-CD38 Mechanism of Action
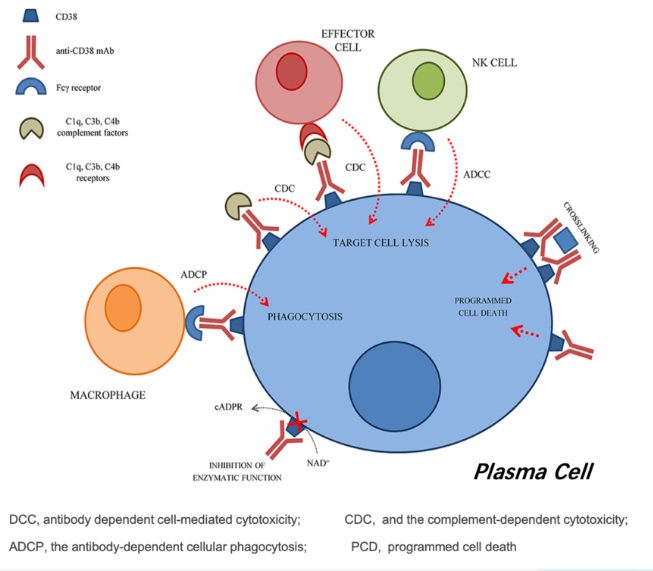
CD38, a type II glycoprotein highly expressed on plasma and natural killer (NK) cells, plays a critical role in the immune response leading to allograft rejection. The anti-CD38 mechanism of action (MOA) involves the targeting of CD38-positive plasma cells responsible for producing donor-specific antibodies, effectively depleting these CD38-positive plasma cells to halt the production of harmful donor-directed and pathologic autoantibodies. At the same time, the anti-CD38 MOA involves the reduction of the number of NK cells, which are known to induce microvascular inflammation and contribute to tissue damage in the renal allograft.
In cases of AMR, it is possible that this dual mechanism of action could play a role in preserving the function of a transplanted organ and improving patient outcomes.
Important Features of CID-103
Preclinical data suggests that CID-103 demonstrates strong antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) activity while reducing complement-dependent cytotoxicity (CDC) activity.
Furthermore, CID-103’s binding to a distinct epitope on the CD38 glycoprotein (R78) reduces red blood cell binding.
The Anti-CD38 Mechanism of Action
Today, CASI is actively working to develop a subcutaneous formulation of CID-103, an innovation that we believe could potentially enhance patient convenience and treatment adherence. We believe a subcutaneous formulation could be especially useful for kidney transplant patients, for example, whose venous access for IV administration is often compromised following years of dialysis.
Other Products
CASI has launched a set of commercial pharmaceutical products in China. These include EVOMELA® (melphalan) for and FOLOTYN® (pralatrexate injection).
CASI OTHER r&D pipeline
CASI has a set of development programs underway for hematologic malignancies & oncology besides CID-103 specifically. These programs are as follows: